| Bright, K; Dienes, B; Keiluweit, M; Rixen, C; Aeppli, M. Climate change impacts on organic carbon cycling in European alpine soils. Soil Biology & Biochemistry, 2025, 210, 109891, doi:10.1016/j.soilbio.2025.109891. |
| Shu, Z; Liu, Q; Dai, Z; Pan, Z; Aeppli, M; Wang, Z. Heterogeneous Photochemical Generation of Hydroxyl Radical in Mineral-Organics Systems: Dual Roles of Iron Oxides. Environmental Science & Technology, 2025, 59 (27), 13820-13831, doi:10.1021/acs.est.5c04440. |
| Lacroix, EM; Gomes, A; Honeyman, AS; Huy, KR; Fendorf, S; Noël, V; Aeppli, M. Soil Carbon Concentration Drives Anoxic Microsites Across Horizons, Textures, and Aggregate Position in a California Grassland. Geoderma, 2025, 454, 117165, doi:10.1016/j.geoderma.2025.117165. |
| Noël, V; Boye, K; Naughton, HR; Lacroix, EM; Aeppli, M; Kumar, N; Fendorf, S; Webb, SM. X-ray chemical imaging for assessing redox microsites within soils and sediments. Frontiers in Environmental Chemistry, 2024, 5, doi:10.3389/fenvc.2024.1329887. |
| Lim, J; Wehmeyer, H; Heffner, T; Aeppli, M; Gu, W; Kim, PJ; Horn, M; Ho, A. Resilience of aerobic methanotrophs in soils; spotlight on the methane sink under agriculture. FEMS Microbiology Ecology, 2024, 100 (3), fiae008, doi:10.1093/femsec/fiae008. |
| Obradović, N; Joshi, P; Arn, S; Aeppli, M; Schroth, MH; Sander, M. Reoxidation of Reduced Peat Organic Matter by Dissolved Oxygen: Combined Laboratory Column-Breakthrough Experiments and In-Field Push-Pull Tests.Journal of Geophysical Research: Biogeosciences, 2023, 128 (11), doi:10.1029/2023JG007640. |
| Lacroix, EM, Aeppli, M; Boye, K; Brodie, E; Fendorf, S; Keiluweit, M; Naughton, HR, Noel Vincent Noël, V; Sihi, D. Consider the Anoxic Microsite: Acknowledging and Appreciating Spatiotemporal Redox Heterogeneity in Soils and Sediments.ACS Earth Space Chemistry, 2023, 7 (9), 1592–1609, doi:10.1021/acsearthspacechem.3c00032. |
| Aeppli, M; Schladow, G; Lezama Pacheco, J S; Fendorf, S. Iron Reduction in Profundal Sediments of Ultraoligotrophic Lake Tahoe under Oxygen-Limited Conditions. Environmental Science & Technology, 2023, 57 (3), 1529-1537, doi:10.1021/acs.est.2c05714. |
| Aeppli, M; Thompson, A; Dewey, C; Fendorf, S. Redox Properties of Solid Phase Electron Acceptors Affect Anaerobic Microbial Respiration under Oxygen-Limited Conditions in Floodplain Soils. Environmental Science & Technology, 2022, 56 (23), 17462-17470, doi:10.1021/acs.est.2c05797. |
| Lopez, AM; Nicolini, CM; Aeppli, M; Luby, SP; Fendorf, S; Forsyth, JE. Assessing Analytical Methods for the Rapid Detection of Lead Adulteration in the Global Spice Market. Environmental Science & Technology, 2022, 56 (23), 16996-17006, doi:10.1021/acs.est.2c03241. |
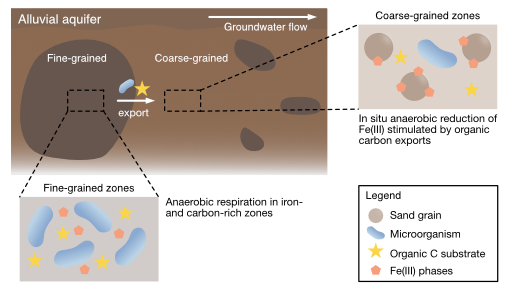
| Aeppli, M; Babey, T; Engel, M; Fendorf, S, Bargar, JR; Boye, K. Export of Organic Carbon From Reduced Fine-Grained Zones Governs Biogeochemical Reactivity in Simulated Aquifer. Environmental Science & Technology, 2022, 56 (4), 2738-2746, doi:10.1021/acs.est.1c04664. |
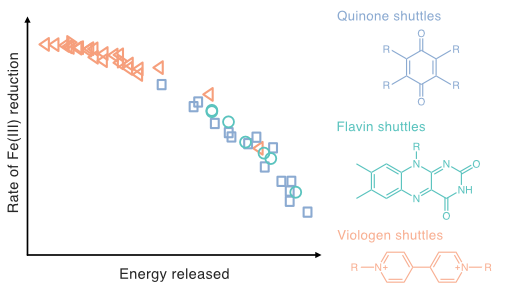
| Aeppli, M; Giroud, S; Vranic, S; Voegelin, A; Hofstetter, TB; Sander, M. Thermodynamic Controls on Rates of Iron Oxide Reduction by Extracellular Electron Shuttles. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119, e2115629119, doi:10.1073/pnas.2115629119. |
| Biswakarma, J; Rushworth, D; Srivastava, G; Singh, G; Kang, K; Das, S; Anantharaman, SB; Aeppli, M; Popp, AL; Bhuyan, DJ. Organizational Level Responses to the COVID-19 Outbreak: Challenges, Strategies and Framework for Academic Institutions. Frontiers in Communication, 2021, 6:573585, doi:10.3389/fcomm.2021.573585. |
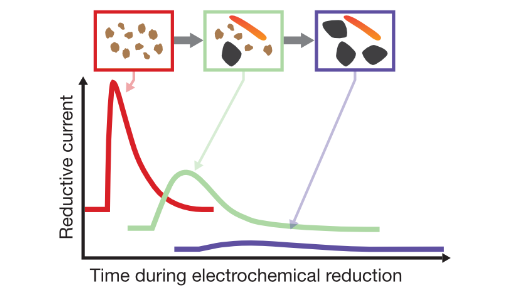
| Aeppli, M; Vranic, S; Kaegi, R; Kretzschmar, R; Brown, AR; Voegelin, A; Hofstetter, TB; Sander, M. Decreases in Iron Oxide Reducibility during Microbial Reductive Dissolution and Transformation of Ferrihydrite. Environmental Science & Technology, 2019, 53 (15), 8736–8746, doi:10.1021/acs.est.9b01299. |
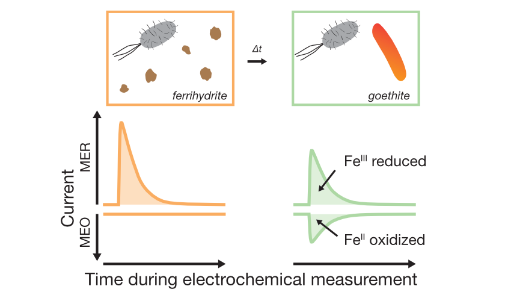
| Aeppli, M; Kaegi, R; Kretzschmar, R; Voegelin, A; Hofstetter, TB; Sander, M. Electrochemical Analysis of Changes in Iron Oxide Reducibility during Abiotic Ferrihydrite Transformation into Goethite and Magnetite. Environmental Science & Technology, 2019, 53 (7), 3568-3578, doi:10.1021/acs.est.8b07190. |
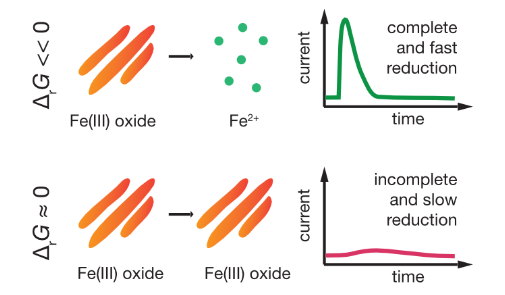
| Aeppli, M; Voegelin, A; Gorski, CA; Hofstetter, TB; Sander, M. Mediated Electrochemical Reduction of Iron (Oxyhydr-)Oxides under Defined Thermodynamic Boundary Conditions. Environmental Science & Technology, 2018, 52 (2), 560-570, doi:10.1021/acs.est.7b04411. |
| Armanious, A; Aeppli, M; Jacak, R; Refardt, D; Sigstam, T; Kohn, T; Sander, M. Viruses at Solid-Water Interfaces: A Systematic Assessment of Interactions Driving Adsorption. Environmental Science & Technology, 2016, 50 (2), 732-743, doi:10.1021/acs.est.5b04644. |
| Franchini, AG; Henneberger, R; Aeppli, M; Zeyer, J. Methane Dynamics in an Alpine Fen: A Field-Based Study on Methanogenic and Methanotrophic Microbial Communities. FEMS Microbiology Ecology, 2015, 91 (3), doi:10.1093/femsec/fiu032. |
| Armanious, A; Aeppli, M; Sander, M. Dissolved Organic Matter Adsorption to Model Surfaces: Adlayer Formation, Properties and Dynamics at the Nanoscale. Environmental Science & Technology, 2014, 48 (16), 9420-9429, doi:10.1021/es5026917. |